Sorry if this has been posted before, seems like a pretty cool idea
what is the exact definition of “functional cure?”
btw, I appreciate the repost as I did not see the other post. thank you!
Hi @Jb007,
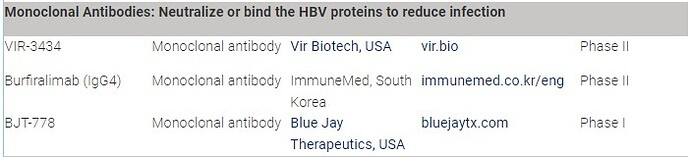
Yes, there are a few companies looking at similar strategies (from Hepatitis B Foundation: Drug Watch):
@catcher.007, an in-depth explanation of what functional cure means is in video form here: What do we mean by Hepatitis B cure? - HBV Cure FAQs
Cheers,
Thomas
Dr. Tu,
thank you so much!
I did not know that we now have different cure level terminologies!
and wow we have so many drugs!
it sure would be nice if we can get one drug to work.
wouldn’t it be faster and effective to have a fewer drugs being worked on instead of so many? It’s like putting fundings and efforts in a few rather than spreading out the fundings and efforts in multiple drugs.
We don’t know which of these are likely to work, so testing a broad range of approaches is the most efficient way at the moment so that we can get closer to a cure. Once a few look promising then they are pushed along from phase I to II and beyond.
TT
I just found this news in the internet and i think this is another hope to cure HBV. You can read article and watch the interview with Carter Keller.
Previously discussed here: GIGA-2339 polyclonal antibody treatment
it’s an interesting approach for sure though!
Let’s hope for the best!
Hi @garry5,
It sounds interesting as many of these trials have. Manufacturers give a lot of hype on the initial results before clinical trials where they get real results. I tend to temper my hope in cases like this, since the actual process is now beginning. Most developments fail at this phase. With that said, I think it will be great if it works and can lead to a cure. The more options we have the better it is for everyone.
Thanks for sharing. Bansah1
From the article: “GIGA-2339 aims to address this gap by using more than 1000 anti-HBV antibodies, replicated from the natural immune response of vaccinated donors.”
One thing that comes to mind is that I’m of the understanding that antibodies from vaccinated people differ significantly to those who develop them due to natural immunity (ie acquiring them from clearing the virus). This is an assumption partly based on prior reading where I saw that hematopoietic stem cell transplants cure chronic HBV but only when the donor has recently acquired then cleared HBV in case studies. Basically it seems that the stem cells start to grow in the recipients liver and produce the antibodies that they learnt from the donor’s environment
I guess it’s harder to get blood samples from that population though. And maybe this will work
Perhaps @availlant could shed some light on this approach? You’re usually great at killing any hope I get ![]() but for good reason (and thank you for your work)
but for good reason (and thank you for your work)
Thanks @Bob for your comments, these are appreciated.
So the problem in achieving functional cure is two fold:
- We have to remove cells in the liver containing integrated HBV DNA. This is because these cells produce most of the HBsAg in chronic HBV infection (in the form of subviral particles) which continue to suppress the immune response.
- These cells will harbour integrated HBV DNA with lots of mutations (as a result of the genetic diversity in HBV infection).
The development of GIGA-2339 is a recognition of the second problem (a genetically and immunologically diverse pool of HBsAg in chronic HBV infection). However, the problem with GIGA-2339 is that the introduction of a very diverse pool of HBsAg reactive antibodies (these will be the antibodies harvested from vaccinated donors) will do nothing directly to target the cccDNA inside cells (which produces new infectious virus) and more importantly, will not result in the removal of cells in the liver containing integrated HBV DNA. Additionally, antibodies only last for about 21 days in the body. To target those liver cells with integrated HBV DNA, we need a broadly HBsAg reactive population of T-cells (which can remove these liver cells).
Interestingly, the introduction of foreign HBsAg antibodies (with a more narrow spectrum of reactivity) is capable of non-specifically stimulating the innate immune response (we have seen this with VIR-4334 - but this is incorrectly called a “vaccinal” effect). This effect can have some impact on cccDNA activity and lowering HBsAg levels but not enough to achieve functional cure. We may observe this effect with GIGA-2339 when it enters trials in infected persons.
Hi all,
Just consolidating all the discussion on this therapy here. Please be sure to search to see if there’s already an existing thread that would be most suitable for discussions, rather than starting a new thread.
Cheers,
Thomas
maybe a naive question. Do liver cells have lifecycle that each cell will die and new cell will grow ? if we can prevent infection of new cells, then will virus and its biomarkers gone with the death of old liver cells ?
There’s a good overview of that here: Example entry: Hepatitis B cccDNA viral reservoirs - stubborn nails in the quest for a complete cure - #14 by DBuddha
So does that mean we would need a diverse approach. Some drugs for clearing cccdna, some for hbsag, and some for integrated hbvdna. And if so, then is there any work going on this approach to handle the hbv.
@availlant i have observed & appreciate from the inner core of my heart that you take your precious time & always explain in detail.
Dear @Nawab,
Welcome to the HBV community and thank you for your comments.
When HBsAg loss occurs, the immune systems of some patients will “wake up” enough to clear integrated HBV DNA on their own. For some other patients, their immune systems will need some help from an immunotherapy to acheive this. In both cases, the evidence of this will be increases levels of ALT in the blood (an enzyme found inside liver cells which gets released into the blood when these cells are destroyed). These are the key events to achieving functional cure of HBV.
It is not possible to clear cccDNA from the liver, but inactivating all the cccDNA that is there may help in the above process. This is routinely achieved by pegylated interferon (pegIFN) - but HBsAg loss during pegIFN therapy is only achieved in patients which already have low HBsAg at the baseline. This is because HBsAg also inhibits immune function that is attempting to control HBV infection.
Currently only NAPs combined with pegIFN achieve high rates of HBsAg loss during therapy, high rates of ALT flares and high rates of HBV functional cure.
Dear @availlant ,
Can you please help with following questions
what you mean by “True HbsAG loss” ?
What is your interpretation of my case?
my last test was done in Dec 2024
- My first test (screening test) showed presence of HbsAg and second test (viral DNA quantification test) showed HbsAg = Non Reactive
- in second test HBV DNA Viral load - (Using Roche Cobas HBV Assay) Detected but below the lower limit of quantification
- HBsAB - Negative ( 6.6 U/L )
- Both tests were done at different labs. My ALT at that time was 166 (Dec 2024)
- My latest blood report - Jan 2025 shows ALT 69
Your post above indicates elevated ALT is a good sign as hep b cells are being cleared. I have been actively working to reduce the ALT through life style changes , food changes , intermittent fasting etc. i can see within a month my ALT came down to 69 from 166.
Thank you