Hello! My name is Xintong, I finished my studies in public health in USYD last year. I was studying the demographic characteristics (sex, age, and ethnicity) of participants in global HBV clinical trials.
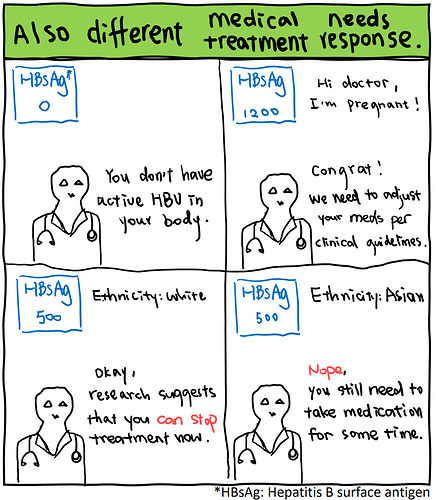
Why do demographic characteristics of trial participants matter? Why should clinical trials be representative of different populations? Just as people have diverse needs in daily life, such as an adult may not fitting into baby clothes or an adolescent requiring more food than a toddler, the needs and treatment responses within and across populations living with HBV are not the same. Clinical trials serve to provide the highest level of evidence to guide clinical practice, assisting health practitioners in deciding treatment plans to suit the needs of individuals from different populations, including those of different sexes, ages, and ethnicities. Insufficient representation of diverse populations in clinical trials poses significant challenges in managing global HBV infections and can harm underrepresented populations, much like an adolescent eating the same portion of food as a toddler daily resulting in poor nutrition.
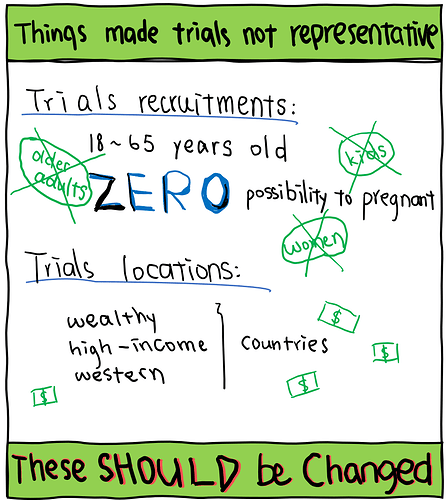
Did HBV clinical trials manage to provide valid evidence to all populations living with HBV? To find out the answer, we reviewed all trials published in the last 15 years around the world. Of the 282 trials we identified, most reported information about participants’ ages and sexes. However, only one-third of these trials reported information about participants’ ethnicities. Even more concerning, less than 10 trials have considered the impact of demographic factors on treatment responses. Trials not providing participant information, or not breaking down results based on demographic characteristics reduce the validity and reliability of evidence for all populations living with HBV.
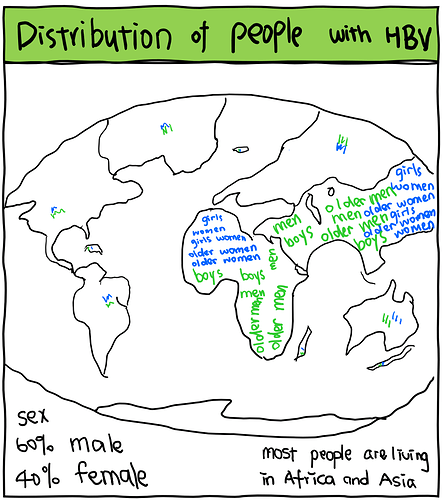
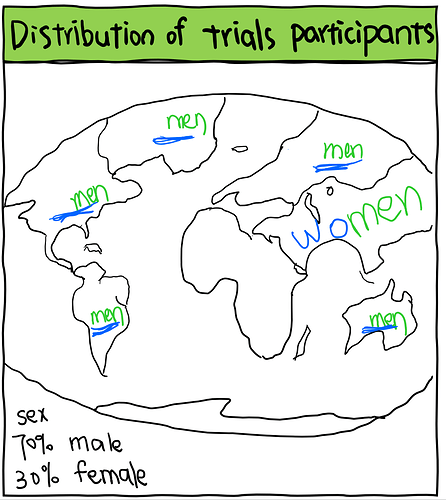
We then investigated the distribution of participants with known demographic characteristics. Compared to the global population living with HBV infection, we concluded that the representation of different populations in these trials is disproportionate - proportions of trial participants not similar to the proportions of people living with HBV in the real world, which is possibly due to exclusion and/or inclusion criteria of these trials.
Our study provides the first quantitative description of participants’ demographic characteristics in recent HBV clinical trials. The identification of under- and over-represented populations in trials informs trial designers and funders about whom to actively recruit in the future, thereby ensuring more valid evidence for all populations living with HBV.
Thank you for reading and looking forward to your questions!